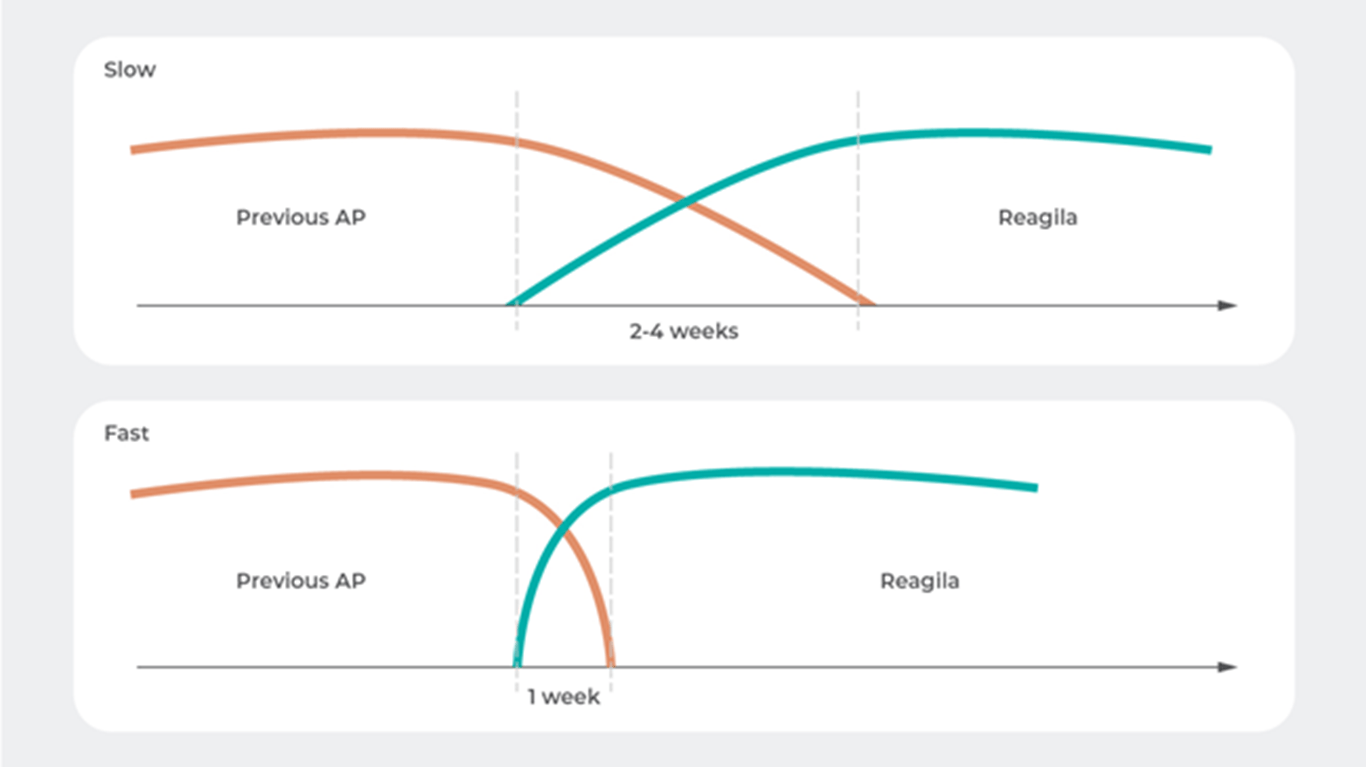
Switching to REAGILA
The ultimate goal in schizophrenia treatment is recovery, which encompasses symptom remission in addition to adequate self-care, improved social and vocational functioning, maintenance of treatment effects, and relapse prevention1.
(COD: 300020/R16. Submitted to AIFA 16/04/2020)
References
- Durgam, S. et al. An evaluation of the safety and efficacy of cariprazine in patients with acute exacerbation of schizophrenia: A phase II, randomized clinical trial. Schizophr. Res. 152, 450–457 (2014).
- Durgam, S. et al. Cariprazine in acute exacerbation of schizophrenia: A fixed-dose, phase 3, randomized, double-blind, placebo- and active-controlled trial. J. Clin. Psychiatry 76, e1574-82 (2015).
- Kane, J. M. et al. Efficacy and Safety of Cariprazine in Acute Exacerbation of Schizophrenia: Results from an International, Phase III Clinical Trial. J. Clin. Psychopharmacol. 35, 367–373 (2015).
- Németh, G. et al. Cariprazine versus risperidone monotherapy for treatment of predominant negative symptoms in patients with schizophrenia: a randomised, double-blind, controlled trial. Lancet 389, 1103–1113 (2017).
- Stahl, S. M. Stahl ’ s Essential Psychopharmacology: Prescriber’s Guide. (Cambridge University Press, 2017).
Essential Psychopharmacology: Prescriber’s Guide, 6th edition
STARTING DOSES FOR REAGILA
STARTING DOSES FOR OUR PRODUCT
(COD: 300020/R15. Submitted to AIFA 16/04/2020) REAGILA is indicated for the treatment of schizophrenia in adult patients. This covers all types of symptoms of
REAGILA AND DRUG INTERACTIONS
CARIPRAZINE AND DRUG INTERACTIONS
(COD: 300020/R18. Submitted to AIFA 16/04/2020) Cariprazine is metabolized into 2 pharmacologically active moieties: desmethyl cariprazine (DCAR) and didesmethy
Contact Med Info
If you have any questions about REAGILA, you can contact our Medical Information team.
GET IN TOUCH